Overview
Overview
The Latin American Neurotrauma Study: Biomarkers in Traumatic Brain Injury (LATINO-BIO-TBI) is an international, multicenter prospective observational research initiative designed to gather data from traumatic brain injury (TBI) patients across healthcare facilities in Latin America and the Caribbean. This project comprises three studies: two targeting Emergency Departments and one focusing on Intensive Care Units.
Serum biomarkers have proven useful for objectively evaluating the severity of TBI and its outcomes. These biomarkers could become key elements in managing TBI patients. While research has predominantly taken place in North America and Europe, there is still limited understanding of how these techniques apply and work in Latin America and the Caribbean.
The LATINO-BIO-TBI study seeks to gather serum levels of Glial Fibrillary Acidic Protein (GFAP) and Ubiquitin C-terminal hydrolase L1 (UCH-L1) from TBI patients in Emergency Departments and Intensive Care Units. This research aims to explore how the serum concentrations of these biomarkers correlate with the severity of the primary injury, their trends throughout the ICU stay, and the resulting functional outcomes.
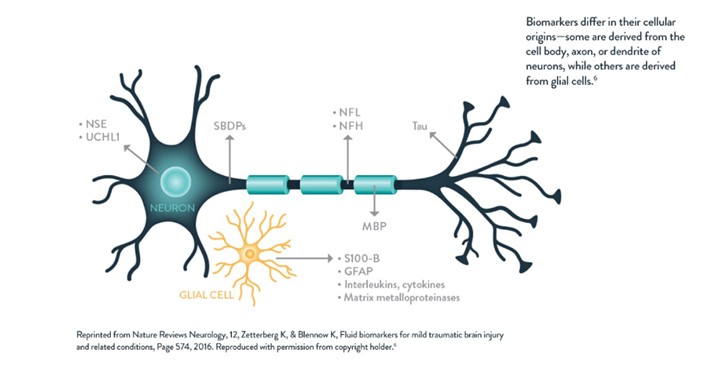
- Ruiz FT, Torrecilla FM, Sánchez MÁA, Gómez IA, Bártulos AV, España FJG. Traumatismo craneoencefálico leve y biomarcadores de lesión cerebral aguda. Rev Esp Urg Emerg. 2024;3:31–6.
- Gordillo-Escobar E, Egea-Guerrero JJ, Rodríguez-Rodríguez A, Murillo-Cabezas F. Utilidad de los biomarcadores en el pronóstico del traumatismo craneoencefálico grave. Med Intensiva . 2016;40(2):105–12. Disponible en: http://dx.doi.org/10.1016/j.medin.2015.11.008
- Calderón Pérez MP, Montosa Ródenas P, Molina Espinoza S. Papel de los biomarcadores GFAP y UCH-L1 en la valoración de un traumatismo craneoencefálico leve en urgencias: una revisión narrativa. AMU. 2024;6(1):50-56.
- Ergun R, Bostanci U, Akdemir G, et al. Prognostic value of serum neuron-specific enolase levels after head injury. Neurol Res. 1998;20(5):418-420.
- Skogseid IM, Nordby HK, Urdal P,et al. Increased serum creatine kinase BB and neuron specific enolase following head injury indicates brain damage. Acta Neurochir. 1992;115:106-111.
- García de Yébenes Prous MJ, Carmona Ortells L. Biomarcadores: cómo lograr su consolidación en práctica clínica. Reumatol Clin [Internet]. 2024;20(7):386–91. Disponible en: http://dx.doi.org/10.1016/j.reuma.2024.05.005
GFAP and UCH-L1 in TBI
GFAP and UCH-L1 in TBIe
Blood-based biomarkers (BBMs) provide quantifiable insights into neuronal, astrocytic, and vascular injury, reflecting the degree of structural brain damage. This information complements the clinical signs and symptoms currently used to diagnose and monitor TBI. Among the many BBMs researched, GFAP and UCH-L1 show the most promise, especially when utilized together.
GFAP is a protein that forms intermediate filaments within the cellular cytoskeleton of astrocytes. It is highly specific to nervous tissue, with serum levels rising in the initial hours following trauma. These levels peak at 20 hours and remain elevated for up to 72 hours, after which they begin to decline.
UCH-L1 is a protein found exclusively in neurons and is one of the most abundant proteins in the brain, accounting for 1-2% of total proteins. Its blood levels begin to rise shortly after trauma; however, it has a short half-life, peaking at 8 hours, after which levels start to decrease.
By combining both BBMs, a marker of glial damage that is highly specific to nervous tissue and remains elevated in the blood for a significant duration is obtained, along with a marker of neuronal injury that increases from the moment of injury and clears more rapidly. Both have proven helpful in the acute phase of TBI, reliably distinguishing between healthy controls and patients with brain injury, predicting abnormalities in diagnostic brain imaging, and forecasting mortality and functional outcomes.
Design
Design
The project will include three planned multicenter observational cohort studies: two will take place in emergency departments and one in intensive care units.
LATINO-BIO-TBI: CT-BIO Emergency Department Study
LATINO-BIO-TBI: CT-BIO Emergency Department Study
This study is a prospective, multicenter, observational clinical trial based on the registered data at LATINO-TBI. Data will be collected to determine the association of acute GFAP and UCH-L1 levels with the primary injury severity of TBI patients in the Latin American and Caribbean regions. Biomarker levels will also be correlated with demographic variables, associated injuries, and prehospital care variables.
- Study design: Prospective cohort study.
- Study setting: Basic and advanced emergency departments at Latin American and Caribbean healthcare centers.
- Study population: Adult patients admitted to the ER with a TBI diagnosis between February 2025 and February 2027 or until the target number of 320 (50% mild TBI / 50% moderate to severe TBI) is reached.
- Sample size: 320 patients.
- Aims:
- Aim 1: Determine acute serum GFAP and UCH-L1 levels and their association with demographic information, hemodynamic markers, imaging findings, neurological state (FOUR score/GCS), and mortality in the emergency department, as well as during the first 24 hours after the event.
- Aim 2: Correlate the serum GFAP and UCH-L1 levels with 24-hour in-hospital mortality, the requirement for neurosurgical interventions, and the association with low GCS confounders (toxicological exam).
LATINO-BIO-TBI: CT-NM-BIO Emergency Department Study
LATINO-BIO-TBI: CT-NM-BIO Emergency Department Study
This study is a prospective, multicenter, observational clinical trial based on the registered data at LATINO-TBI. Data will be collected to determine the association of acute GFAP and UCH-L1 levels with the primary injury severity of TBI patients in the Latin American and Caribbean regions. Biomarker levels will also be correlated with demographic variables, associated injuries, and prehospital care variables. We will include patients with TBI diagnoses admitted to medium-to-high complexity ED facilities. Patients with pre-existing neurological disorders will be excluded. The primary outcome will be the correlation with injury severity (clinical, non-invasive monitoring and imaging). Secondary outcomes will include emergency room mortality (from the event up to 24 hours after) and the need for neurosurgical interventions.
- Study design: Prospective cohort study.
- Study setting: Medium-to-high complexity emergency departments at Latin American and Caribbean healthcare centers.
- Study population: Adult patients admitted to the ER with a TBI diagnosis between February 2025 and February 2027 or until the target number of 320 (50% mild TBI / 50% moderate to severe TBI) is reached.
- Sample size: 320 patients.
- Aims:
- Aim 1: Determine acute serum GFAP and UCH-L1 levels and their association with demographic information, hemodynamic markers, imaging findings, non-invasive neuromonitoring [optic nerve sheath diameter (ONSD), automated pupillometry, transcranial Doppler (TCD), and ICP waveform pattern)], neurological state (FOUR score/GCS), and mortality in the emergency department, as well as during the first 24 hours after the event.
- Aim 2: Correlate the serum GFAP and UCH-L1 levels and non-invasive neuromonitoring findings with 24-hour in-hospital mortality, the requirement for neurosurgical interventions, and the association with low GCS confounders (toxicological exam).
LATINO BIO-TBI: CT-NM-BIO Intensive Care Units Study
LATINO BIO-TBI: CT-NM-BIO Intensive Care Units Study
This study is a prospective, multicenter, observational clinical trial based on registered data from LATINO-TBI. Data will be collected to determine the association between acute and chronic GFAP and UCH-L1 levels and the functional outcomes of TBI patients in the Latin American and Caribbean regions. Biomarker levels will also be correlated with hemodynamic markers, both invasive and noninvasive neuromonitoring, and imaging findings. We will include patients diagnosed with TBI admitted to the ICU through referrals from high-complexity trauma medical centers.
Patients with pre-existing neurological disorders will be excluded. The primary outcome will be functional outcomes rated by the Glasgow Outcome Scale Extended (GOSE) at discharge and at 3, 6, 12, and 24 months. Secondary outcomes will include in-hospital mortality, the frequency and type of neurosurgical interventions, medical and surgical complications, the length of ICU and hospital stays (in days), and dichotomized GOSE scores across multiple thresholds.
- Study design: Prospective cohort study.
- Study setting: Intensive Care Units (ICU) of high-complexity trauma referral medical centers from the Latin American and Caribbean regions.
- Study population: Adult patients admitted to the ICUs with a TBI diagnosis between February 2025 and February 2026 or until the target number of 80 is reached.
- Sample size: 80 patients.
- Aims:
- Aim 1: Determine an acute and chronic timeline for serum GFAP and UCH-L1 levels and regress them with demographic information, hemodynamic markers, imaging findings, neurological state (FOUR score/GCS), invasive neuromonitoring (ICP value, ICP waveform pattern, and PbTiO2), non-invasive neuromonitoring (optic nerve sheath diameter (ONSD), automated pupillometry, transcranial Doppler (TCD), and ICP waveform pattern), and functional outcomes (GOSE) at medical discharge and 3, 6, 12, and 24 months post-discharge.
- Aim 2: Correlate the serum GFAP and UCH-L1 levels with in-hospital mortality, the frequency and type of neurosurgical interventions, medical and surgical complications, and the length of ICU and hospital stays (in days).

